On April 8, 2015, Canon announced the launch of the 4K new concept digital camera XC10. The product has the ability to capture 4K high quality video and high quality still images. As a product for professionals and video shooters, whether it is a promotional film, a documentary, or a news gathering or as a digital cinema shooting machine that requires first-class portability, the XC10 has a good face in response to a wide range of shooting needs. applicability.
In order to achieve excellent image quality in 4K video, the XC10 uses four Canon's latest technologies: the newly developed high-sensitivity 1.0-type CMOS image sensor, 10x optical zoom 4K camera lens, and high-performance DIGIC DV 5 image. Processing platform, as well as the exclusive development of the XF-AVC video format.
The XC10 adopts Intra Frame (intra-frame compression) 4K video compression mode, which can achieve a recording rate of up to 305 Mbps bit rate, achieving high data compression ratio without sacrificing image quality, thereby further reducing the amount of data. In addition, the new 4K new concept camera also uses the same video recording format as the Canon EOS System (CINEMA EOS System), which controls overexposure or underexposure even in very bright or dark areas. At the very least, achieving excellent image quality. The XC10 is also equipped with a CFast 2.0 specification memory card slot for recording 4K video without the need for external storage devices.
The Canon XC10 can also capture high-resolution still images. The machine is equipped with a 12-megapixel CMOS image sensor with a maximum sensitivity of ISO 20,000. It is equipped with a mechanical shutter for shooting high-speed moving objects. It can eliminate the skew caused by the rolling shutter. In addition, this 4K new concept camera also supports 4K frame cutting, users can capture about 8.92 million pixels of still images from 4 frames of motion images at 30 frames per second.
The XC10 has a compact, lightweight body design with a body size of approximately 125 (W) x 102 (H) x 122 (D) mm and a weight of approximately 930 grams. In addition, it uses a reversible liquid crystal display that can be flipped up by about 90° and flipped down by about 65°, while the grip can also be rotated about 90° forward and backward, respectively. The XC10 is designed to allow users to shoot from a variety of different angles of view and angle, which is essential for recording video in high-mobility shooting scenes.
The Canon XC10 4K new concept camera is scheduled to begin sales in mid-June 2015.

You can find us here:
App download:
Or scan the QR code above
Sina Weibo: @装备COOL http://weibo.com/lvyoumallofficial
WeChat public platform: search for "zhuangbeiku"
The first time to receive the most practical equipment dry goods!

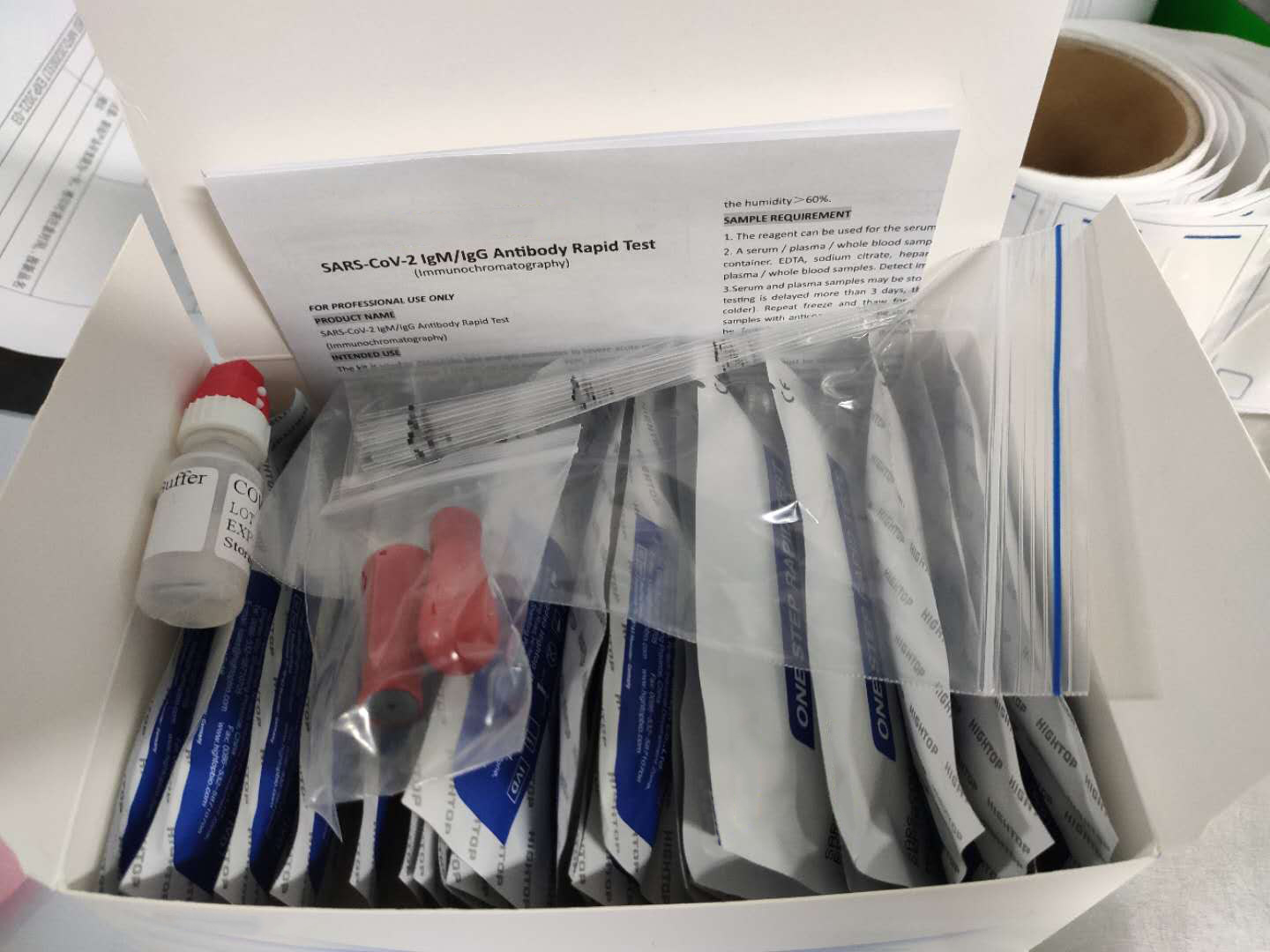
Description about Coronavirus(COVID-19)test Test
The Coronavirus(COVID-19)test is a solid phase immunochromatographic assay used in the rapid, qualitative and differential detection of IgG and IgM antibodies to the 2019 novel coronavirus in human whole blood, serum or plasma. The Coronavirus(COVID-19)test is intended for professionals within highly complex settings and or pharmacies. The test delivers rapid results between 2 and 10 minutes from individuals having signs and symptoms of SARS-CoV-2 infection
What conditions to do the Coronavirus(COVID-19)test?
1.Breating difficulty
2.skin sores, which may become a skin ulcer that heals very slowly
3.stuffy nose, runny nose, and nosebledds.
4.swallowing difficulty
TEST OVERVIEW for the Coronavirus(COVID-19)test
- Utilizes human whole blood, serum, or plasma
- Used in rapid, qualitative and differential detection of IgG and IgM antibodies
- Delivers clinical results between 2 and 10 minutes at the point-of-care
- Visual interpretation of results
- No special equipment needed
PERFORMANCE CHARACTERISTICS
The COVID-19 lgG/lgM Rapid Test (Whole Blood/Serum/Plasma) has been evaluated with the 126 samples obtained from patients exhibiting pneumonia or respiratory symptoms. The results were compared to Fluorescent Real Time Polymerase Chain Reaction (RT-PCR) or clinical diagnosis (including chest Computed Tomography and clinical signs etc.) of [Diagnosis and treatment of novel coronavirus pneumonia."
IgG/IgM Rapid Test results compared to SARS-CoV-2 RT-PCR results:
| COVID-19 RT-PCR Assay | Coronavirus(COVID-19)test | ||
| Positive | Negative | Total | |
| Positive | 103 | 9 | 112 |
| Negative | 0 | 14 | 14 |
| Total | 103 | 23 | 126 |
| 95% CI | |||
| Sensitivity | 103/112 | 91.9% | (85.3% – 96.3%) |
| Specificity | 14/14 | 100% | (76.8% – 100%) |
| Overall | 117/126 | 92.8% | (86.9% – 96.7%) |
The sensitivity of lgM only test is 89.2% (100/112) and specificity is 100% (14/14).
The sensitivity of lgG & IgM test is 91.9% (103/112) and specificity is 100% (14/14).
RT-PCR Method testing was included as clinical site method. Nucleic acid was detected by probe fluorescence PCR. This product contained primers and probes (ORF1ab gene and N gene strains of coronavirus) and internal controls (housekeeping gene beta Globin gene sequences) used in RT-PCR for the in vitro qualitative detection of SARS-CoV-2 RNA in nasopharyngeal swab specimens. The novel coronavirus2019-ncov specific probe was respectively labeled with FAM fluorescence and ROX fluorescence, and the internal standard gene was labeled with VIC fluorescence. The minimum detection limit of the fluorescent RT-PCR assay is 10 copies/reaction.
LIMITATIONS
- Use fresh samples only.
- This test has not been reviewed by the FDA.
- Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus.
- Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals.
- Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
- Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E. Some specimens containing unusually high titer of heterophile antibodies or rheumatoid factor may affect expected results.
-
Not for the screening of donated blood
Rapid Test,Coronavirus Test Kit,Coronavirus Test,Covid-19 Test
Dongguan Smart Furniture Co.,Ltd , https://www.smtfurniture.com